Publications 2013-2018
2018
Peterson, A., Kaabel, S., Kahn, I.,Pehk, T., Aav, R., Adamson, J. Unsubstituted Oxacalix[n]arenes (n=4 and 8): A Conformational Study in Solution and Solid State and Interaction Studies with Aromatic Guests ChemistrySelect, 2018 3, 9091−9095. DOI: 10.1002/slct.201801590
Aav, R., Mishra, K. A. The breaking of symmetry leads to chirality in cucurbituril-type hosts, Symmetry 2018, 10, 98, doi:10.3390/sym10040098. (Invited) Special issue: Chiral Auxiliaries and Chirogenesis

Adamson, J.; Nazarski, R. B.; Jarvet, J.; Pehk, T.; Aav, R. Shortfall of B3LYP in Reproducing NMR JCH Couplings in Some Isomeric Epoxy Structures with Strong Stereoelectronic Effects: A Benchmark Study on DFT Functionals. ChemPhysChem 2018, 19, 631 – 642. DOI : 10.1002/cphc.201701125
Kaabel, S.; Aav, R. Templating Effects in the Dynamic Chemistry of Cucurbiturils and Hemicucurbiturils. Isr. J. Chem. 2018, 58, 296 –313. DOI: 10.1002/ijch.201700106 (Invited) Special issue: Cucurbiturils and Related Cavitands, Cover Picture Isr. J. Chem. 2018, 58, 186

2017
Kaabel, S.; Adamson, J.; Topić, F.; Kiesilä, A.; Kalenius, E.; Öeren, M.; Reimund, M.; Prigorchenko, E.; Lõokene, A.; Reich, H. J.; Rissanen, K.; Aav, R. (2017). Chiral hemicucurbit[8]uril as an anion receptor: selectivity to size, shape and charge distribution. Chemical Science, 8 (3), 2184−2190. doi: 10.1039/C6SC05058A.
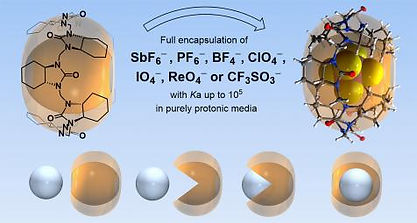
Fomitšenko, M.; Peterson, A.; Reile, I.; Cong, H.; Kaabel, K.; Prigorchenko, E.; Järving I.; Aav R. (2017). A quantitative method for analysis of mixtures of homologues and stereoisomers of hemicucurbiturils that allows us to follow their formation and stability. New Journal of Chemistry, 41, 2490−2497.10.1039/C6NJ03050E.

N Konrad, D Kananovich, R Aav, V Borovkov, Spectroscopic Study of (all-R, R)-cyclohexanohemicucurbit [8] uril and Its Host-Guest Supramolecular Hexafluorophosphate Complexes, Multidisciplinary Digital Publishing Institute Proceedings 2017, 2 (1), 64.
Kaasik, M.; Kaabel, S.; Kriis, K.; Järving, I.; Aav, R.; Rissanen, K.; Kanger, T. Synthesis and Characterisation of Chiral Triazole-Based Halogen-Bond Donors: Halogen Bonds in the Solid State and in Solution. Chemistry - A European Journal, 2017, 23 (30), 7337−7344.10.1002/chem.201700618.
Aav, R.; Kaabel, S.; Fomitšenko, M. Cucurbiturils: Synthesis, Structures, Formation Mechanisms, and Nomenclature.
In Comprehensive Supramolecular Chemistry II; Atwood, J. L., Ed.; Elsevier: Oxford, 2017; Volume 3, pp. 203–220, ISBN 978-0-12-803199-5. (Invited)

2016
Aav, R. Chiral molecular containers hemicucurbiturils – Are they symmetric or asymmetric? Symmetry: Culture and Science, 2016, 28 (2), 167−170.
2015
Prigorchenko, E.; Öeren, M.; Kaabel, S.; Fomitšenko, M.; Reile, I.; Järving, I.; Tamm, T.; Topic, F.; Rissanen, K.; Aav, R. Template-controlled synthesis of chiral cyclohexylhemicucurbit[8]uril. Chemical Communications, 2015, 51, 10921−10924.

Parve, J.; Reile, I.; Aid, T.; Kudrjašova, M.; Müürisepp, A.-M.; Vallikivi, I.; Villo, L.; Aav, R.; Pehk, T.; Vares, L.; Parve, O. Lipase-catalyzed stereoresolution of long-chain 1,2-alkanediols: a screening of preferable reaction conditions. Journal of Molecular Catalysis B: Enzymatic, 2015,116, 60−69.10.1016/j.molcatb.2015.03.006.
2014
Kõllo, M.; Aav, R.; Tamp, S; Jarvet, J.; Lopp, M. Asymmetric synthesis of the 2,2,3-trisubstituted cyclopentanone, D-ring fragment of 9,11-secosterols. Tetrahedron, 2014, 70 (38), 6723−6727.10.1016/j.tet.2014.07.079.
Öeren, M.; Shmatova, E.; Tamm, T.; Aav, R. Computational and ion mobility MS study of (all-S)-cyclohexylhemicucurbit[6]uril structure and complexes. Physical Chemistry Chemical Physics, 2014, 36, 19198−19205.10.1039/c4cp02202e.
.png)
Fomitšenko, M.; Shmatova, E.; Öeren, M.; Järving, I.; Aav, R. New homologues of chiral cyclohexylhemicucurbit[n]urils. Supramolecular Chemistry, 2014, 26 (9), 698−703.10.1080/10610278.2014.926362.
2013
Aav, R.; Shmatova, E.; Reile, I.; Borissova, M.; Topic, F.; Rissanen, K. New Chiral Cyclohexylhemicucurbit[6]uril. Organic Letters, 2013,15 (14), 3786−3789.10.1021/ol401766a.

Kõllo, M.; Kudrjašova, M.; Kulp, M.; Aav, R. Methylphosphonic acid as a 31P-NMR standard for the quantitative determination of phosphorus in carbonated beverages. Analytical methods, 2013, 5 (16), 4005−4009.10.1039/C3AY40743H.

